Details of the Drug
General Information of Drug (ID: DMND304)
| Drug Name |
Miltefosine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
HDPC; HePC; Hexadecylphosphocholine; Hexadecylphosphorylcholine; Impavido; Miltefosina; Miltefosinum; Miltex; Baxter Oncology brand of miltefosine; Baxter brand of miltefosine; Miltefosin C; Prasfarma brand of miltefosine; D 18506; IN1227; D-18506; H-1850; M-7200; Miltefosina [INN-Spanish]; Miltefosine (INN); Miltefosine[INN:BAN]; Miltefosinum [INN-Latin]; N-Hexadecylphosphorylcholine; TF-002; Choline hydroxide, hexadecyl hydrogen phosphate, inner salt; Choline, hexadecyl hydrogen phosphate, inner salt; Hexadecyl 2-(trimethylazaniumyl)ethyl phosphate; Choline phosphate, hexadecyl ester, hydroxide, inner salt; Choline phosphate, hexadecyl ester, hydroxide, inner salt (6CI); Hexadecyl 2-(trimethyl-.lambda.~5~-azanyl)ethyl hydrogen phosphate; Ethanaminium, 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethyl-, hydroxide, inner salt; Ethanaminium, 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethyl-, hydroxide, innner salt; 1-Hexadecylphosphorylcholine; 2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethylethanaminium hydroxide, inner salt; 3,5-Dioxa-4-phosphaunacosan-1-aminium, 4-hydroxy-N,N,N-trimethyl-, hydroxide, inner salt, 4-oxide
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
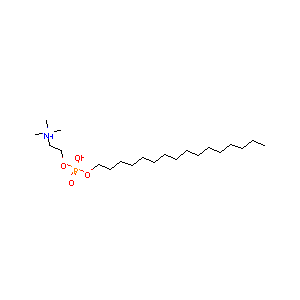 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 407.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.7 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 20 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic urticaria | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Miltefosine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Miltefosine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | ||||
| 3 | Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005-2009). Rev Iberoam Micol. 2009 Mar 31;26(1):15-22. | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg. 2006 Dec;100 Suppl 1:S17-20. | ||||
| 6 | MDR1 causes resistance to the antitumour drug miltefosine. Br J Cancer. 2001 May 18;84(10):1405-11. doi: 10.1054/bjoc.2001.1776. | ||||
| 7 | Dimethylsphingosine and miltefosine induce apoptosis in lung adenocarcinoma A549?cells in a synergistic manner. Chem Biol Interact. 2019 Sep 1;310:108731. doi: 10.1016/j.cbi.2019.108731. Epub 2019 Jun 29. | ||||
| 8 | Product Information. Impavido (miltefosine). Paladin Therapeutics Inc, Wilmington, DE. | ||||
| 9 | Bailey DG, Arnold JMO, Spence JD "Grapefruit juice and drugs - how significant is the interaction." Clin Pharmacokinet 26 (1994): 91-8. [PMID: 8162660] | ||||
